Many elements in nature have isotopes, some occurring naturally, some artificially produced, some radioactive, and some non-radioactive.
Like everything in this world, an isotope is an atom, the smallest unit of matter that has all the chemical properties of the same element, and a form of a chemical element with special physical properties.
On the periodic table you can find different chemical elements.

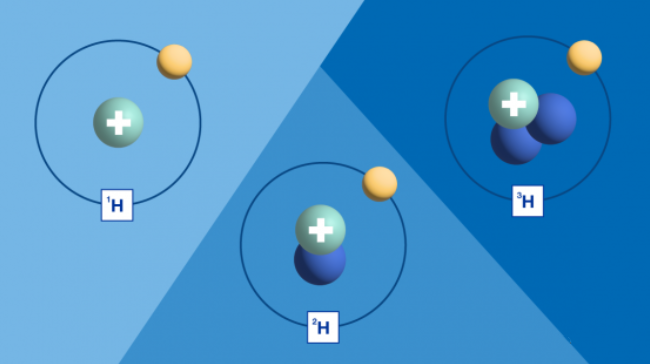
Different elements are distinguished by the number of protons, neutrons, and electrons it has. Atoms of every chemical element have a definite and identical number of protons and electrons, but the key is neutrons – the number of which is variable.
Atoms with the same number of protons but different numbers of neutrons are called isotopes. They have almost the same chemical properties but different masses and therefore different physical properties. Stable isotopes do not emit radiation, while unstable isotopes do. The latter are called radioactive isotopes.

Nuclear techniques are used to measure the amounts and proportions of isotopes in a substance and use this information to trace its origin, circulation and origin. These measurements help experts understand everything from terrestrial and aquatic systems, the amount of specific vitamins absorbed by the body, or the amount of fertilizer absorbed by plants.
What is an isotopic signature?
An isotope signature is a collection of ratios between the amounts of various isotopes of an element in a sample.
Isotopic signatures are often called fingerprints because of their similarity to a person’s fingerprints and are used for tracking and tracing. They are found in water, land, plants and animals. By tracking these fingerprints, scientists can assess:
①Migration of species on land and water
②Animal food chain and dietary changes
③Geographical and plant sources of food
④The age and quality of water bodies (including groundwater aquifers)
⑤Sources of water and air pollution

For example, the naturally occurring isotope carbon-14 found in water can be used to understand the age of water and other organic materials.
Stable isotopes
On the periodic table, the first 80 elements have stable isotopes. The properties of stable isotopes make them useful in understanding and managing water and land resources. They are also used in environmental research, nutritional assessment and forensic science.
For example, the isotope of hydrogen is a naturally occurring stable isotope. By measuring their amounts and proportions in water samples, scientists can determine the age and origin of water, understand its circulation and confirm its origin. This is called isotope hydrology.

Stable isotopes are used to study land, humans, animals, insects and plants. For example, isotopes are used to map the migration paths of butterflies and help protect resources in their breeding environments.

Stable isotopes can also be used in agriculture. Using bio-nitrogen fertilizers labeled with the stable isotope nitrogen-15 (15N), scientists can track and determine the effectiveness of fertilizer uptake by crops. This is crucial – plants need to take in nitrogen and convert it into essential proteins. Scientists use 15N to determine how much fertilizer a crop needs to achieve maximum yield.
Radioactive isotopes

There are over 3,000 known radioactive isotopes. They are unstable forms of elements. These radioisotopes emit varying degrees of radiation, making them useful in medicine, industry, agriculture, radiopharmaceutical science, industrial applications, environmental tracking, and biological research.
Radioisotopes are produced safely by humans in research reactors and accelerators. One use of radioisotopes is in the treatment of cancer and chronic diseases using radionuclides to treat cancer cells in a safe and effective way. Other uses include producing safer health products by removing or neutralizing chemicals, bacteria and toxins that pose hazards.

Radioactive isotopes: Isotopes that emit rays spontaneously are called radioactive isotopes.
(The radioactive isotopes mentioned below all refer to artificial radioactive isotopes.)
There are generally three methods for preparing artificial radioactive isotopes:
Produced in nuclear reactors and used to prepare neutron-rich isotopes, referred to as heap-illuminating isotopes;
Prepared by charged particle accelerators, mostly used in the production of neutron-poor isotopes, referred to as accelerator isotopes;
Isotopes are separated and extracted from the nuclear fuel reprocessing liquid. This isotope is usually called a split isotope.



